A team of University of California, Irvine researchers have published the first comprehensive overview of the major changes that occur in mammalian skin cells as they prepare to heal wounds. Results from the study provide a blueprint for future investigation into pathological conditions associated with poor wound healing, such as in diabetic patients.
“This study is the first comprehensive dissection of the major changes in cellular heterogeneity from a normal state to wound healing in skin,” said Xing Dai, Ph.D., a professor of biological chemistry and dermatology in the UCI School of Medicine, and senior author. “This work also showcases the collaborative efforts between biologists, mathematician and physicists at UCI, with support from the National Institute of Arthritis & Musculoskeletal & Skin Diseases-funded UCI Skin Biology Resource-based Center and the NSF-Simons Center for Multiscale Cell Fate Research.
The study, titled, “Defining epidermal basal cell states during skin homeostasis and wound healing using single-cell transcriptomics,” was published this week in Cell Reports.
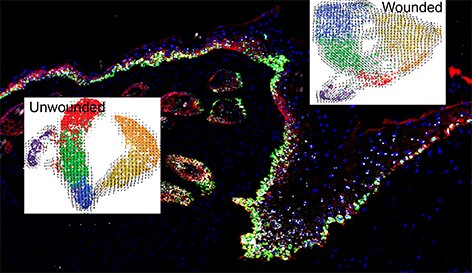
“Our research uncovered at least four distinct transcriptional states in the epidermal basal layer as part of a ‘hierarchical-lineage’ model of the epidermal homeostasis, or stable state of the skin, clarifying a long-term debate in the skin stem cell field,” said Dai.
Using single-cell RNA sequencing coupled with RNAScope and fluorescence lifetime imaging, the team identified three non-proliferative and one proliferative basal cell state in homeostatic skin that differ in metabolic preference and become spatially partitioned during wound re-epithelialization, which is the process by which the skin and mucous membranes replace superficial epithelial cells damaged or lost in a wound.
Epithelial tissue maintenance is driven by resident stem cells, the proliferation and differentiation dynamics of which need to be tailored to the tissue’s homeostatic and regenerative needs. However, our understanding of tissue-specific cellular dynamics in vivo at single-cell and tissue scales is often very limited.
“Our study lays a foundation for future investigation into the adult epidermis, specifically how the skin is maintained and how it can robustly regenerate itself upon injury,” said Dai.
News Source: www.medicalxpress.com
More information: Daniel Haensel et al, Defining Epidermal Basal Cell States during Skin Homeostasis and Wound Healing Using Single-Cell Transcriptomics, Cell Reports (2020). DOI: 10.1016/j.celrep.2020.02.091
