A new driver of inflammation in aging: Mitochondrial RNA leakage

A major new study authored by Stella Victorelli and Madeline Eppard and led by Joao Passos, of Mayo Clinic, uncovers a previously unknown mechanism driving chronic inflammation in aging.
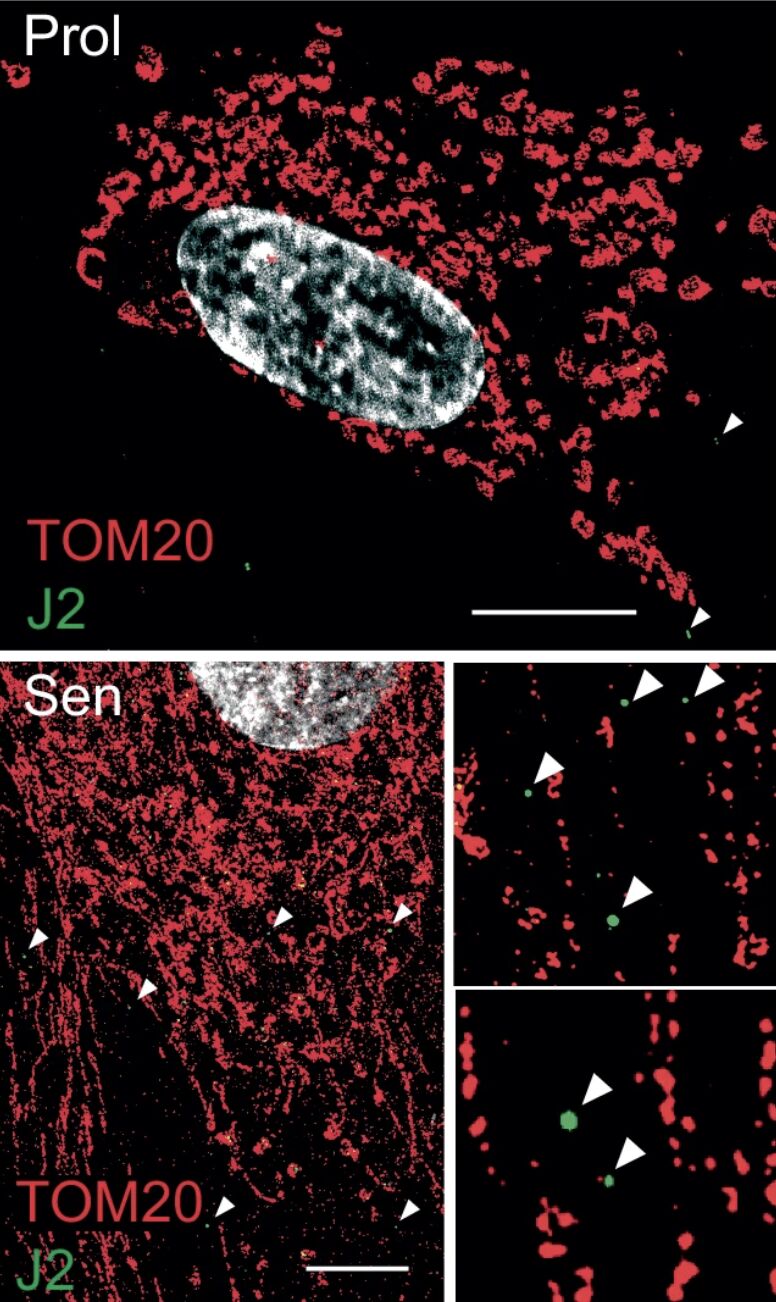
The research indicates that mitochondrial RNA (mtRNA) leaking into the cytosol of senescent cells acts as a key trigger for the senescence-associated secretory phenotype (SASP), a significant factor contributing to tissue dysfunction and age-related diseases.
What the study found:
- Senescent cells accumulate mtRNA in the cytosol, where it activates innate immune RNA sensors RIG-I and MDA5, leading to Mitochondrial Antiviral Signaling protein (MAVS) aggregation and inflammatory signaling.
- The study identifies BAX and BAK-dependent mitochondrial membrane permeabilization as the mechanism allowing mtRNA leakage. Genetic deletion of BAX/BAK suppresses SASP both in vitro and in vivo.
- In a mouse model of metabolic dysfunction–associated steatohepatitis (MASH), inhibiting this mtRNA-RNA sensing axis reduced liver inflammation and fibrosis markers, demonstrating physiological relevance.
Why it matters:
This work expands the aging paradigm beyond mitochondrial DNA by identifying mtRNA as a potent endogenous danger signal. It reveals a new mitochondria-to-cytosol signaling axis that fuels chronic inflammation in aging and age-related disease. It highlights RNA sensing, MAVS, and mitochondrial permeability as promising therapeutic targets to mitigate inflammaging without eliminating senescent cells.
Read the full open-access study: Click here
We are also pleased to remind you that the World Mitochondria Society (WMS), in collaboration with the International Society of Microbiota (ISM), is organizing the Targeting Longevity World Congress 2026, taking place on April 8–9 in Berlin, Germany. Read more
Did you like the news ? Please share it with your circle.